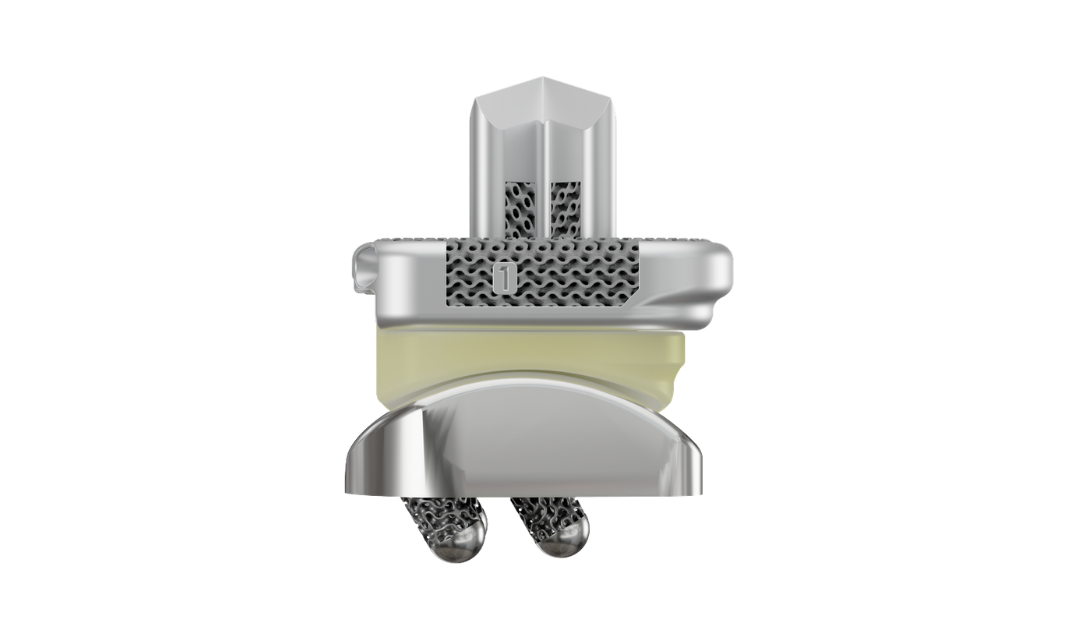
3D-Printed Solutions for Orthopedic Surgery
At restor3d, we create personalized medical products to improve patient outcomes. By combining 3D printing, biomaterials, and biomechanics, we aim to expand patient-specific musculoskeletal care. Explore our products below.
Knee
Explore Products
Personalized joint replacement solutions tailored to your practice needs and your patient’s anatomy.
Hip
Explore Products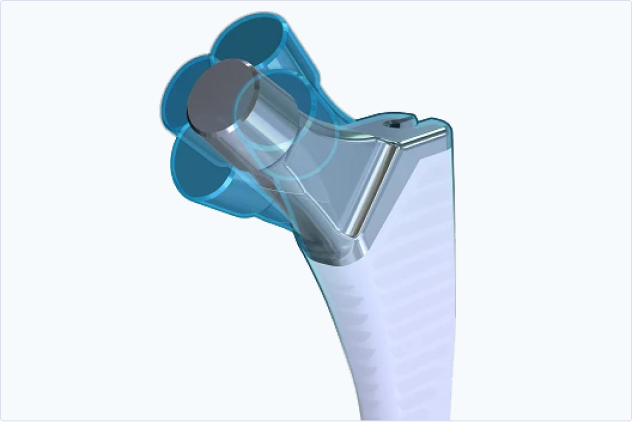
3D image-to-implant hip replacement system providing intuitive surgical plans and instruments.
Digital Solutions
r3id | Personalized Surgery
Our digital platform allows surgeons to create and track cases efficiently and collaborate with restor3d’s Design Engineering Team to maximize patient-specific surgical outcomes.